In-situ analysis of unstable products and online spectral monitoring have become the only research methods
In a certain nitration reaction, strong acids such as nitric acid needs to be used to nitrate raw materials to generate nitration products. The nitration product of this reaction is unstable and easily decomposes. In order to obtain the target product, the entire reaction needs to be carried out in an environment of -60°C. If offline laboratory techniques such as chromatography, mass spectrometry, and nuclear magnetic resonance are used to analyze the product, the product may decompose during the analysis process and accurate information about the reaction cannot be obtained. Using online spectroscopy technology for in-situ real-time monitoring, the content variation of product and the progress of the reaction are clear at a glance. In the study of such reactions containing unstable components, online monitoring technology is almost the only effective research technique.
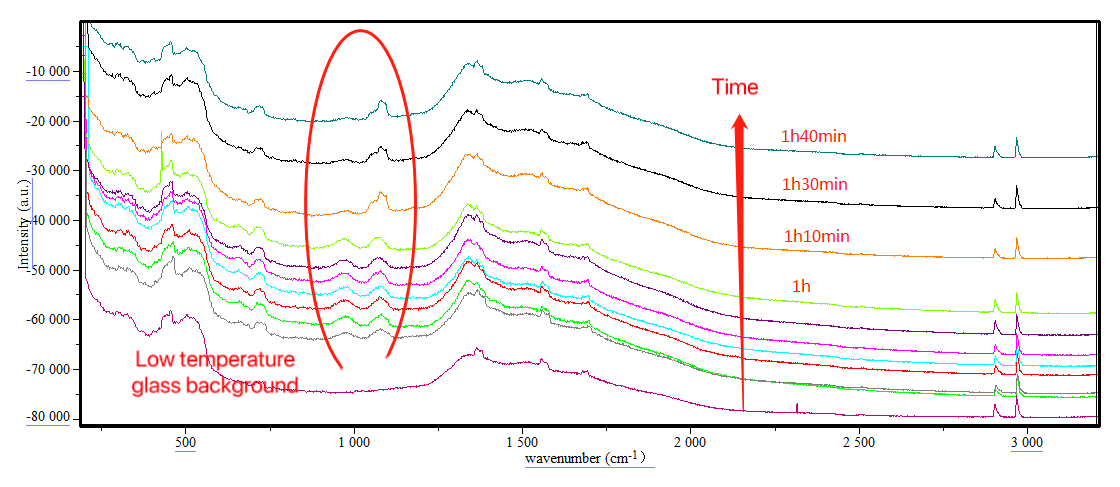
The picture above records the real-time online monitoring of the nitrification reaction. The characteristic peaks of the product at positions 954 and 1076 cm-1 show a clear process of enhancement and decrease over time, which suggests that too long a reaction time will lead to the decomposition of nitration products. On the other hand, the peak area of the characteristic peak reflects the product content in the system. From the online monitoring data, it can be seen that the product content is the highest when the reaction proceeds to 40 minutes, suggesting that 40 minutes is the optimal reaction end point.
Post time: Jan-10-2024

