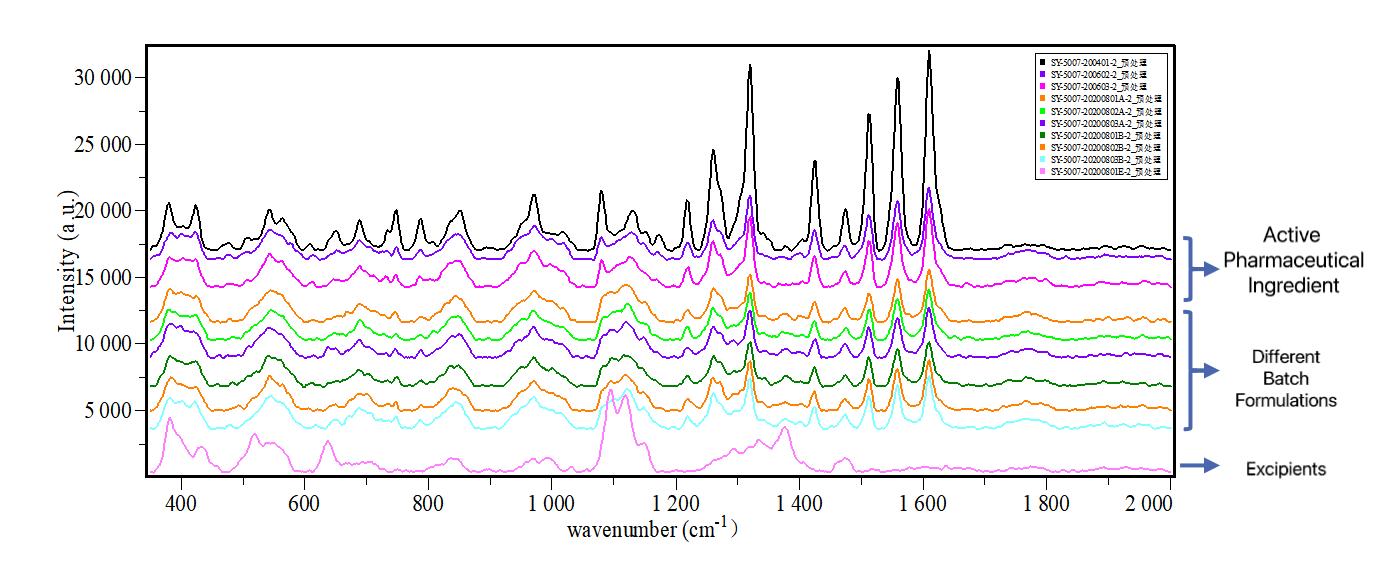
Online Raman quickly determines the consistency of multiple batches of formulations with the crystalline form of active pharmaceutical ingredients.
Online monitoring provides faster results for target crystal testing, continuous data prompts reaction mechanisms and endpoints, providing optimization, directions.
Different crystal forms of the same drug may exhibit significant differences in appearance, solubility, melting point, dissolution rate, bioavailability, etc., thereby affecting the stability, bioavailability, and efficacy of the drug. Therefore, it is necessary to confirm the presence of the target crystal form during drug synthesis and formulation processes.
In the process of developing a new drug, it is necessary to monitor in real-time the crystalline phase composition of the drug in the synthesis reaction solution. This is done to optimize the process and ensure that the drug's target crystalline phase is synthesized. Raman spectroscopy can be used for in-situ monitoring, providing real-time analysis of the crystalline phase composition in the drug synthesis reaction, especially suitable for comprehensive analysis of polymorphic and amorphous API-containing systems.
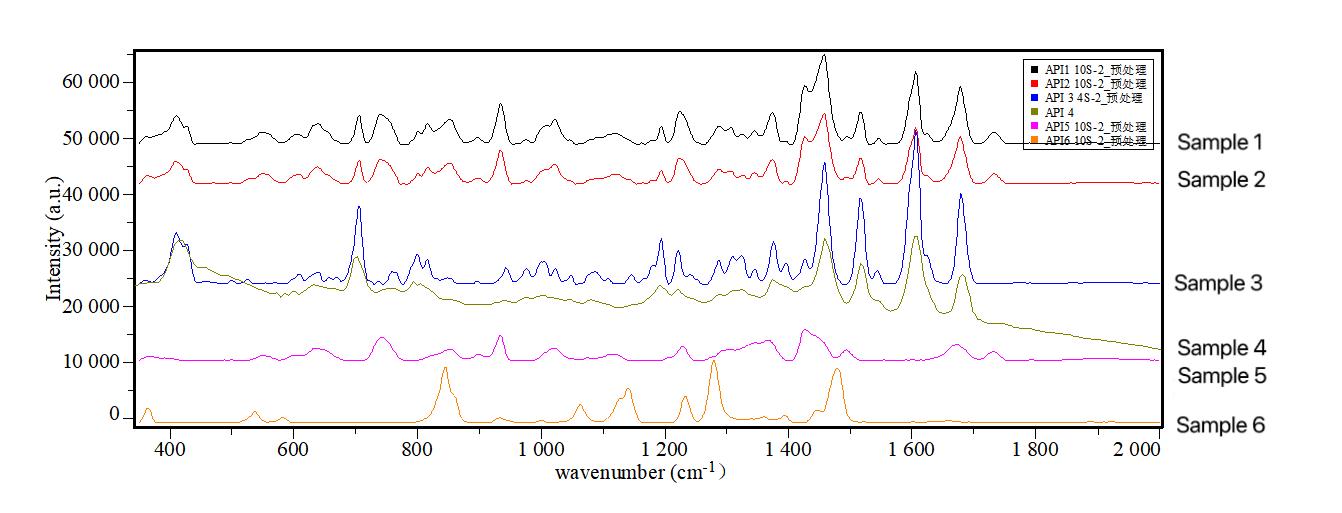
Online Raman spectroscopy streamlines crystal phase screening for diverse reaction conditions, as illustrated by a pharmaceutical company's testing of formulation batches. The results confirmed alignment with the active pharmaceutical ingredient, demonstrating successful research. Prior limitations using XRD and other lab instruments led to data constraints and extended development cycles. Another case highlighted successful real-time differentiation of crystal phase transformations in six different processes, providing immediate insights into product outcomes.
Online Raman quickly determines the crystalline phase transformation results under different reaction conditions.
Post time: Dec-26-2023

