In a highly corrosive environment, online spectroscopy monitoring becomes an effective research method.
Lithium bis(fluorosulfonyl)amide (LiFSI) can be used as an additive for lithium-ion battery electrolytes, with advantages such as high energy density, thermal stability, and safety. The future demand is becoming more evident, making it a hotspot in new energy industry material research.
The synthesis process of LiFSI involves fluoridation. Dichlorosulfonyl amide reacts with HF, where the Cl in the molecular structure is replaced by F, producing bis(fluorosulfonyl)amide. During the process, intermediate products that have not been fully substituted are generated. The reaction conditions are stringent: HF is highly corrosive and extremely toxic; reactions occur under high temperature and pressure, making the process highly dangerous.
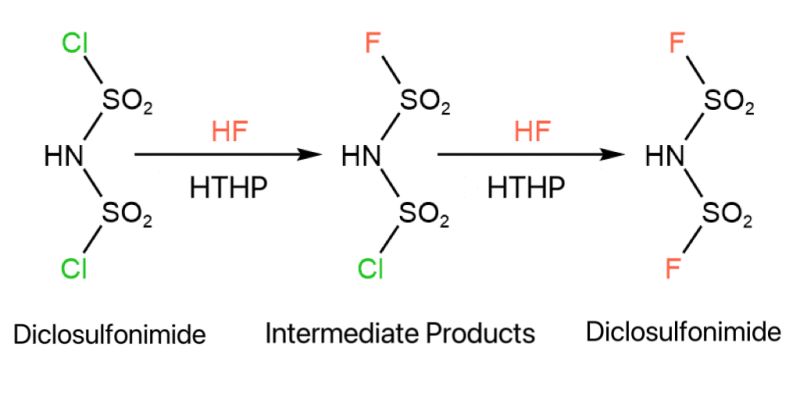
At present, much research on this reaction focuses on finding the optimal reaction conditions to maximize product yield. The only offline detection technique available for all components is the F nuclear magnetic resonance (NMR) spectrum. The detection process is extremely complex, time-consuming, and dangerous. Throughout the substitution reaction, which lasts for several hours, pressure must be released and samples taken every 10-30 minutes. These samples are then tested with F NMR to determine the content of intermediate products and raw materials. The development cycle is lengthy, sampling is complex, and the sampling process also affects the reaction, making the test data unrepresentative.
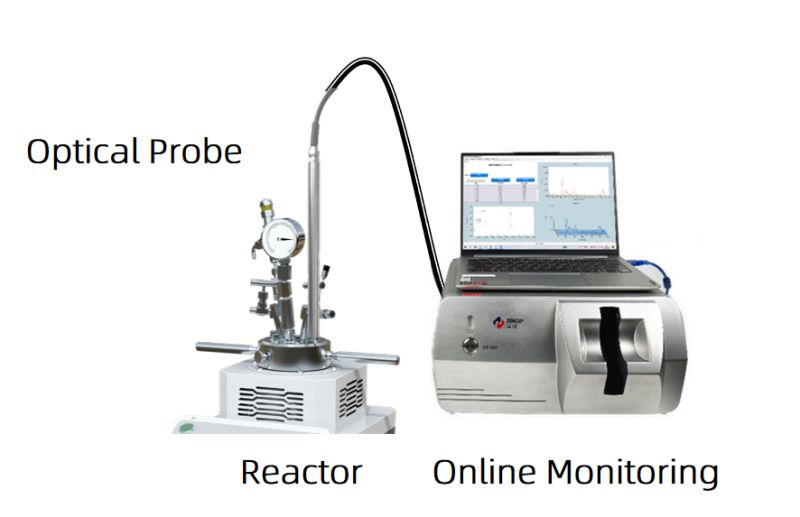
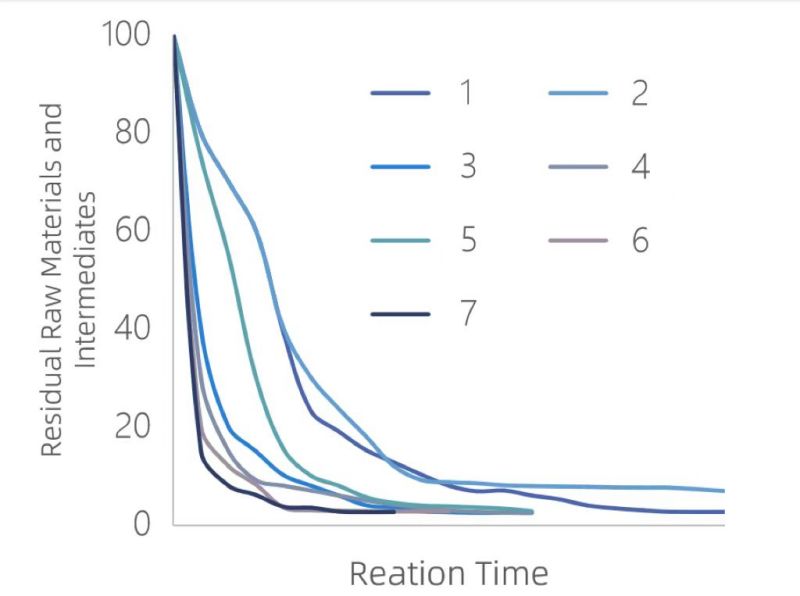
However, online monitoring technology can perfectly address the limitations of offline monitoring. In process optimization, online spectroscopy can be used to monitor the real-time in-situ concentrations of reactants, intermediate products, and products. The immersion probe directly reaches below the liquid surface in the reaction kettle. The probe can withstand corrosion from materials like HF, hydrochloric acid, and chlorosulfonic acid, and can tolerate up to 200°C temperatures and 15 MPa pressure. The left graph shows the online monitoring of the reactants and intermediate products under seven process parameters. Under parameter 7, the raw materials are consumed the fastest, and the reaction is completed the earliest, making it the best reaction condition.
Post time: Nov-23-2023

